Publications
My highlights
Below are some highlights of my recent work. You can find a complete list of my publications at the end of this page or on Google Scholar.
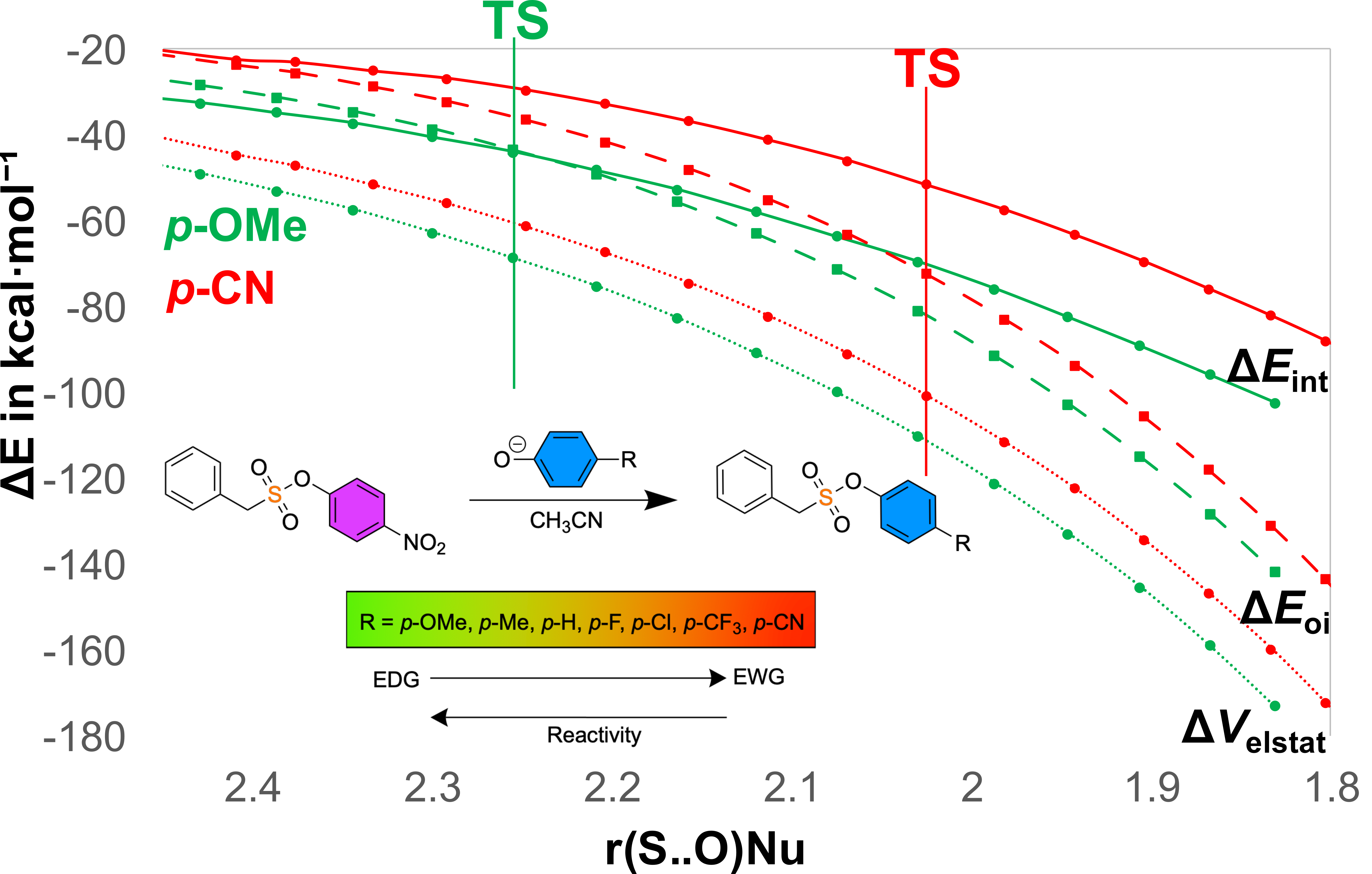
Can enthalpy alone explain reactivity in SuPhenEx reactions? By isolating enthalpic contributions, we show that in the gas phase, reactivity is driven by orbital interactions, tunable via electronic substituents. In polar solvents, enthalpic control shifts toward electrostatic stabilization, shaped by solvent-induced charge distributions. This deeper understanding offers a clear path to designing more efficient S(VI) substitution reactions.
Akash Krishna, Pau Besalu, F. Matthias Bickelhaupt, Guanna Li, and Han Zuilhof
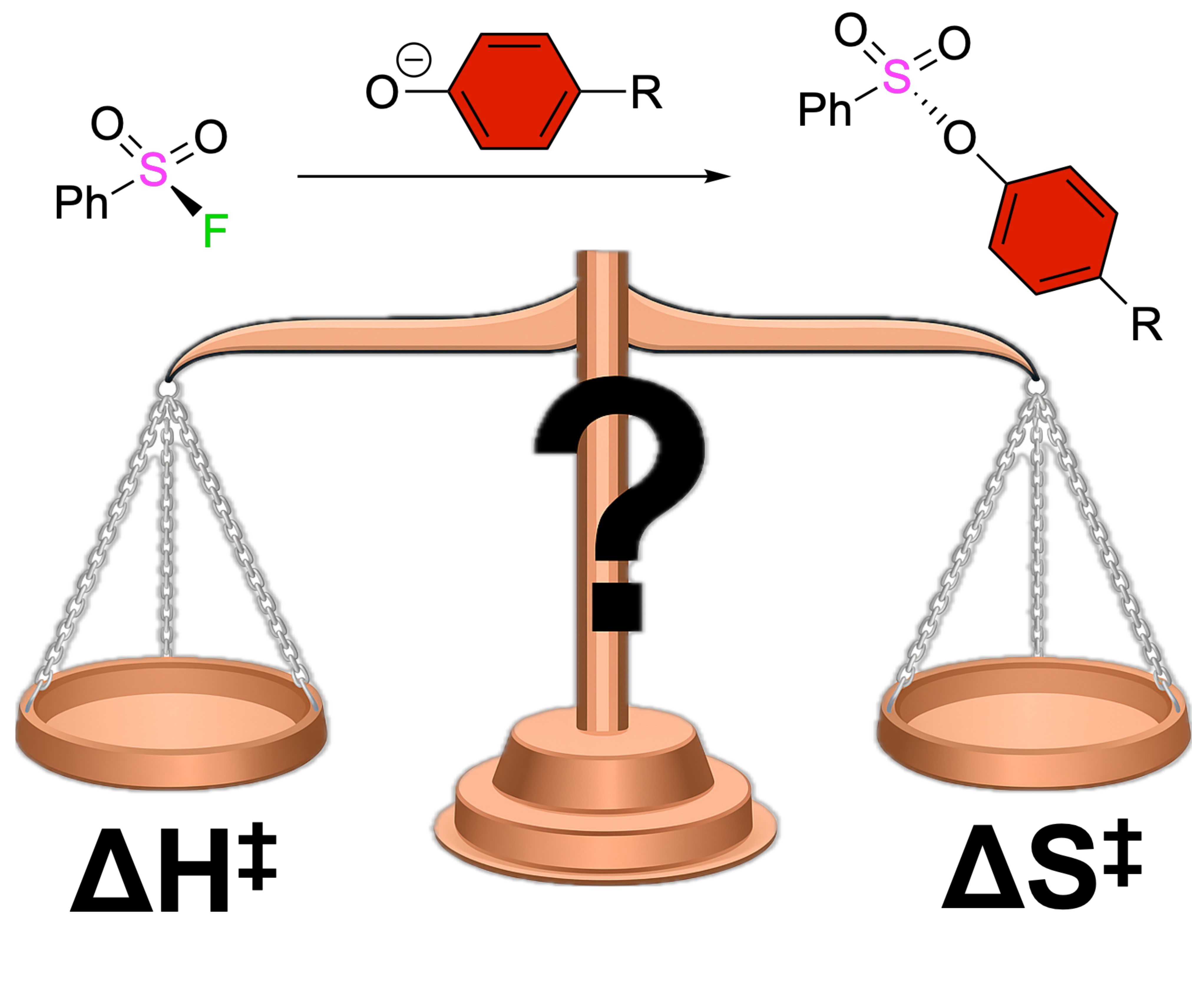
For years, chemists have focused on lowering activation enthalpy (ΔH‡) to control the rate of click reactions, but our study of SuFEx and SuPhenEx reactions reveals that activation entropy (ΔS‡) plays the dominant role. This challenges a long-held assumption and suggests that understanding molecular disorder may be the real key to designing more efficient and sustainable chemistry.
Yumei Zhu, Akash Krishna, Yang Chao, Xixi Li, Sidharam P. Pujari, Guanna Li, Hong Huang, Hongxia Zhao, Jiajia Dong, Han Zuilhof
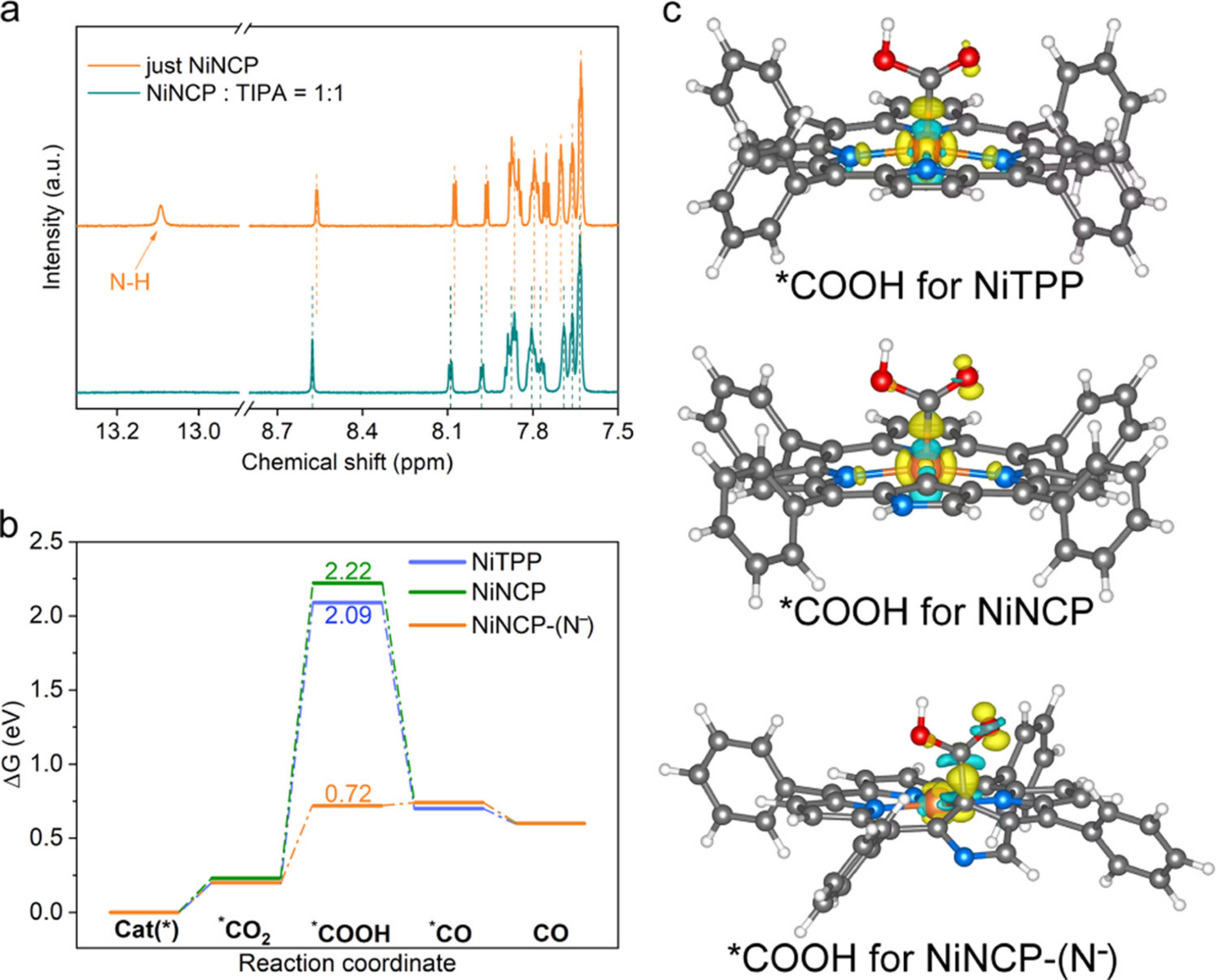
Can ligands do more than just support the metal? In Ni(II) N-confused porphyrin (NiNCP), the ligand’s acidic N–H enables a nonclassical pathway where it directly activates CO₂, achieving a TON of 217,000 with 98% CO selectivity, reshaping how we think about metal–ligand synergy in catalyst design.
Huihong Yuan, Akash Krishna, Zhihe Wei, Yanhui Su, Jinzhou Chen, Wei Hua, Zhangyi Zheng, Daqi Song, Qiaoqiao Mu, Weiyi Pan, Long Xiao, Jin Yan, Guanna Li, Wenjun Yang, Zhao Deng, and Yang Peng
Journal of the American Chemical Society 2024 146 (15), 10550-10558
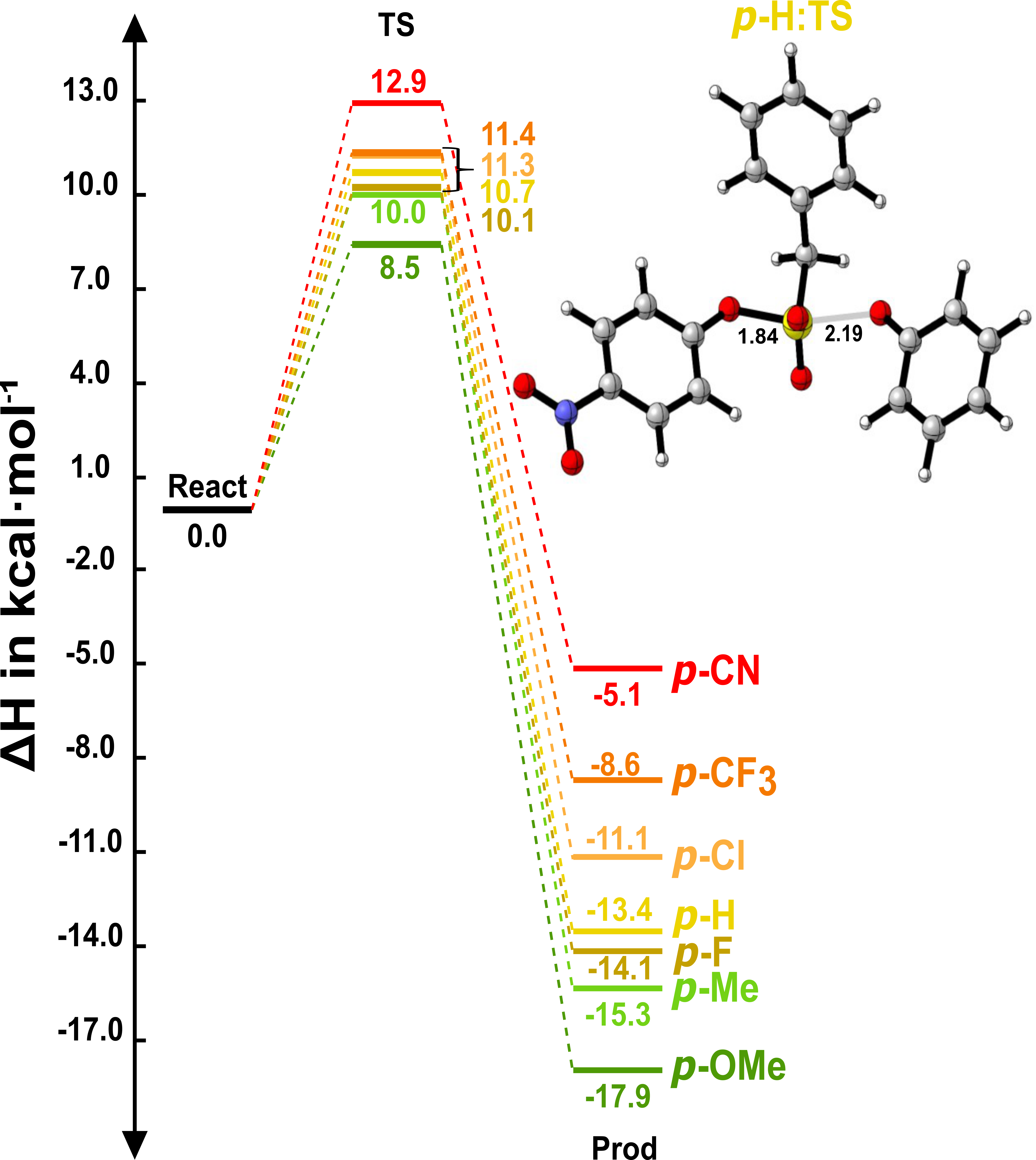
What really drives bond formation in SuPhenEx reactions? By generalizing S(VI) exchange across a broad range of phenols and using DFT with Energy Decomposition Analysis, we move beyond total energies to reveal the key interactions behind reactivity, a story I was excited to share at IUPAC/CHAINS 2023 in The Hague.
A. Krishna, G. Li, H. Zuilhof
Book of abstracts: connecting chemical worlds. IUPAC, p. 307 O46-0783 (2023)
What if click chemistry could be both dynamic and enantiospecific? SuPhenEx, a fast, room-temperature S(VI)–phenolate exchange, achieves this with broad functional group tolerance, enabling access to both enantiomers from a single precursor while avoiding racemization and fluoride related issues, opening new paths in asymmetric synthesis and degradable polymer design
Yang Chao, Akash Krishna, Dr. Muthusamy Subramaniam, Dr. Dong-Dong Liang, Dr. Sidharam P. Pujari, Prof. Andrew C.-H. Sue, Dr. Guanna Li, Dr. Fedor M. Miloserdov, Prof. Dr. Han Zuilhof
Full List of Publications
-
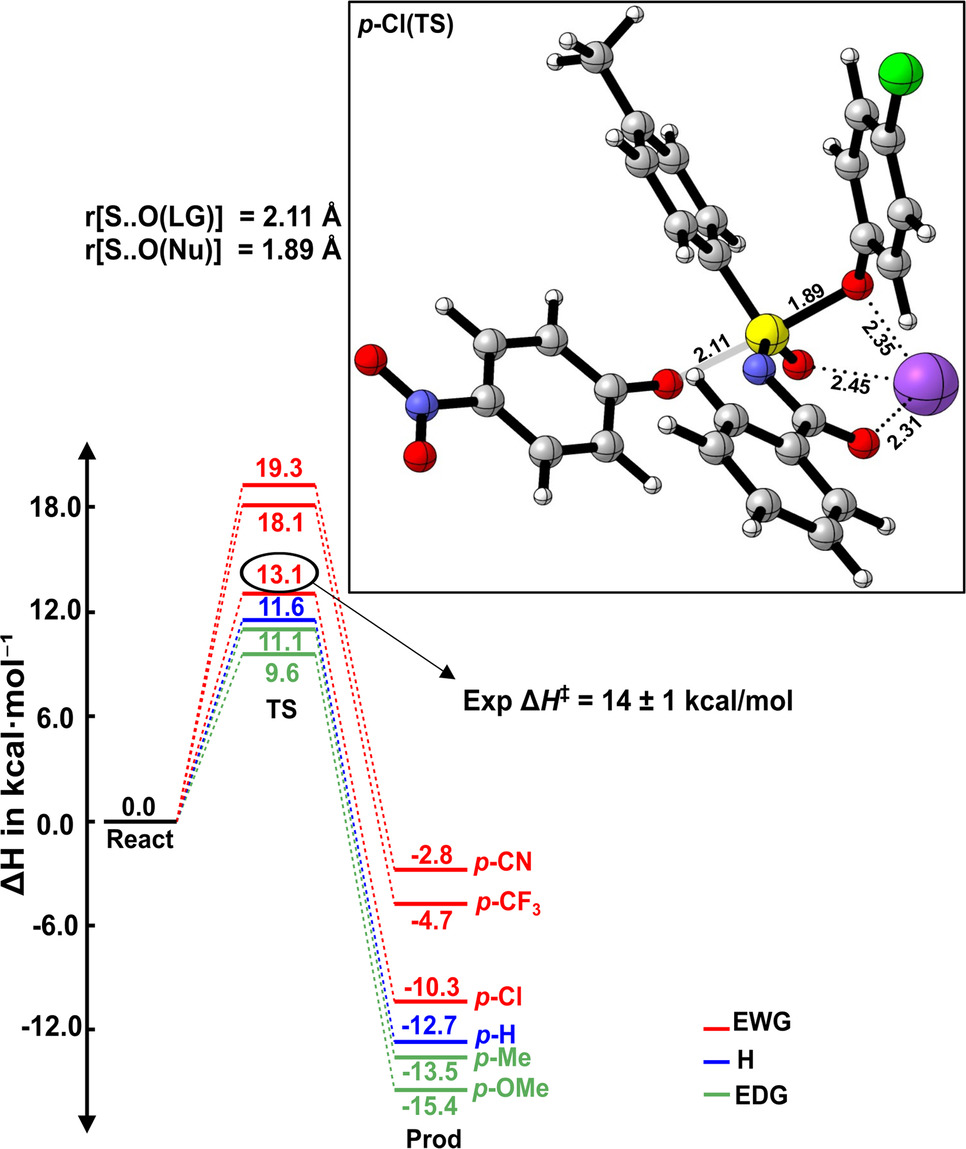
Substituent Effects on Sulfur Phenolate Exchange Reactions: Reactivity and Bonding Analysis - Submitted
Akash Krishna, Pau Besalu, F. Matthias Bickelhaupt, Guanna Li, and Han Zuilhof
EurChemJ 2025 -
Multimodal S(VI) Exchange Click Reactions Derived from SF2 Moieties: Comparative Kinetics and Stereochemistry of SuFEx and SuPhenEx Reactions
Yumei Zhu, Akash Krishna, Yang Chao, Xixi Li, Sidharam P. Pujari, Guanna Li, Hong Huang, Hongxia Zhao, Jiajia Dong, Han Zuilhof
J. Org. Chem. 2025 -
Ligand-Bound CO2 as a Nonclassical Route toward Efficient Photocatalytic CO2 Reduction with a Ni N-Confused Porphyrin
Huihong Yuan, Akash Krishna, Zhihe Wei, Yanhui Su, Jinzhou Chen, Wei Hua, Zhangyi Zheng, Daqi Song, Qiaoqiao Mu, Weiyi Pan, Long Xiao, Jin Yan, Guanna Li, Wenjun Yang, Zhao Deng, and Yang Peng
Journal of the American Chemical Society 2024 146 (15), 10550-10558 -
Another Potential Click Reaction: SuPhenEx – A Computational Insight
A. Krishna, G. Li, H. Zuilhof
Book of abstracts: connecting chemical worlds. IUPAC, p. 307 O46-0783 (2023) -
Sulfur–Phenolate Exchange: SuFEx-Derived Dynamic Covalent Reactions and Degradation of SuFEx Polymers
Yang Chao, Akash Krishna, Dr. Muthusamy Subramaniam, Dr. Dong-Dong Liang, Dr. Sidharam P. Pujari, Prof. Andrew C.-H. Sue, Dr. Guanna Li, Dr. Fedor M. Miloserdov, Prof. Dr. Han Zuilhof
Angew. Chem. Int. Ed. 2022, 61, e202207456 -
Chitosan nanoparticles as a promising tool in nanomedicine with particular emphasis on oncological treatment
Javad Sharifi-Rad, Cristina Quispe, Monica Butnariu, Lia Sanda Rotariu, Oksana Sytar, Simona Sestito, Simona Rapposelli, Muhammad Akram, Mehwish Iqbal, Akash Krishna, Nanjangud Venkatesh Anil Kumar, Susana S. Braga, Susana M. Cardoso, Karolina Jafernik, Halina Ekiert, Natália Cruz-Martins, Agnieszka Szopa, Marcelo Villagran, Lorena Mardones, Miquel Martorell, Anca Oana Docea & Daniela Calina
Cancer Cell Int 21, 318 (2021) -
Evaluation of Antibacterial Properties of Metal Ions [Cu2+, Ni2+, and Ag+] on Escherichia Coli.
Vishal Reddy, Akash Krishna, Prajwal B. G., Rajeshwar Reddy, R. M. Shrihari Bharadwaj, Sahana, P., Ujwala N., Maithri S., Praveena B. and Vyshnavi V. Rao*
WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Volume 8, Issue 8, 1645-1658 (2019)